Scientists Develop New Electrolytes for Low-Temperature Lithium Metal Batteries
Source:金属研究所英文网站(2014改版)
27 06, 2024
Source:金属研究所英文网站(2014改版) 06 27, 2024 | 【 A A A 】 | 【Print】 【Close】
The development of electric vehicles, large-scale energy storage, polar research, deep space exploration has presented higher demands for the energy density and low-temperature performance of energy storage batteries. In recent years, lithium metal batteries with high-specific-capacity of lithium metal anode have become one of the most promising high-energy-density batteries. However, in the carbonate electrolytes, solvent molecules interact strongly with Li+, which consequently hampers the migration of Li+ and the stability of the lithium metal interface. This limitation restricts the application of lithium metal batteries in low-temperature environments.
The research team led by Prof. LI Feng from Institute of Metal Research, Chinese Academy of Sciences (IMR, CAS) proposed a new electrolyte design strategy for regulating the energy of oxygen bonding in the solvent to achieve exceptional performance of lithium metal batteries even under low-temperature conditions. This work was published as a supplementary cover article in Journal of the American Chemical Society.
In the study, the energy of oxygen bonding in solvent was found to be closely associated with ionic coordination and interfacial transport. The impact of different oxygen bonding, such as sulfone (S=O), ester (C=O), and ether (C-O) on the structure and temperature adaptability of the electrolyte was elucidated. Through extensive screening, tetrahydrofuran-based ether solvents with weak oxygen bonding were selected. Based on this foundation, the interaction between Li+ and solvents was further attenuated by hydrogen bonding between fluorinated solvent and ether solvent molecules.
This strategy significantly accelerates the desolvation process of Li+ and reduces the side effects of solvents on the interfacial transport and stability. The lithium metal batteries exhibited a high reversibility with 100% capacity retention after 150 cycles at room temperature, -20 °C and -40 °C. This is one of the most stable low-temperature lithium metal batteries reported in the literature. The practical Ah-level battery exhibited excellent performance with this new electrolyte.This strategy offers a novel approach for developing electrolytes of low-temperature batteries.
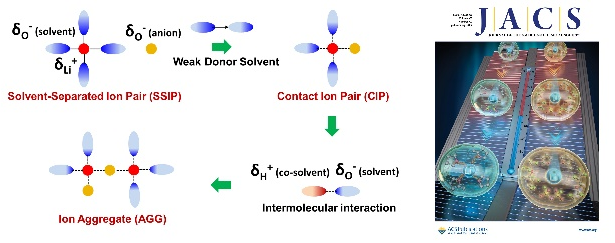
Mechanism of electrolyte design strategy and cover image (Image by IMR)
Contact:
LI Feng
Institute of Metal Research, Chinese Academy of Sciences
Phone:86-24-83970065
Email: fli@imr.ac.cn
