Scientists Unveil Pitting Corrosion Mechanism of Borated Stainless Steel in Wet Storage Environment of Spent Nuclear Fuels
Ensuring the safe storage of spent nuclear fuels (SNFs) is a vital guarantee for the sustainable development of nuclear power. Borated stainless steels (BSSs) are promising materials for SNF storage applications due to the favorable neutron absorption properties of B element. However, the corrosion mechanism of BSS in wet storage environment is still not sufficiently understood.
The research team led by Prof. WANG Jianqiang from Institute of Metal Research, Chinese Academy of Sciences (IMR, CAS) proposed a “Cr depletion-microgalvanic synergetic mechanism” for the corrosion behavior of BSS in the wet storage environment and significantly improved the corrosion resistance of BSS based on this mechanism. This work was published in the Acta Materialia.
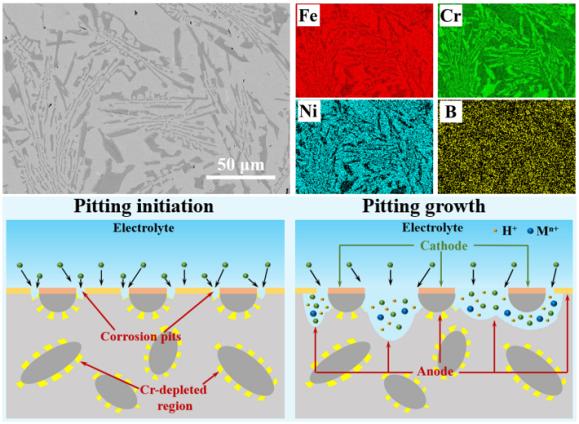
The BSS is composed of a γ austenitic matrix and (Cr, Fe)2B second phase. The pitting corrosion resistance of the BSS was closely associated with the synergistic effect of the pit initiation induced by localized Cr depletion and the pit stable growth induced by microgalvanic effect, causing the pitting corrosion preferentially occurred at the (Cr, Fe)2B/matrix interface. A higher B content increased the density and width while reduced the Cr contents of the Cr-depleted regions, enhancing the susceptibility to pitting initiation. Meanwhile, the strengthened micro-galvanic effect between the (Cr, Fe)2B phase and γ matrix further facilitated the stable pit growth. As for the effect of the wet storage environment, at lower Cl-/H3BO3 ratio, the first-principles calculations indicated that the competitive adsorption of B(OH)4- to Cl- inhibited pitting initiation. On the contrary, the acidizing effect became the dominant factor deteriorating the corrosion resistance in the environment containing high Cl-/H3BO3 ratio. Ultimately, based on the new mechanism, the corrosion resistance of the BSS was significantly improved by tailoring the characterizations of the (Cr, Fe)2B phase.
This study was done in collaboration with Prof. CHEN Xingqiu from IMR, CAS. Prof. YU Bo from Shenyang Research Institute of Foundry and Dr. LIU Zhiwen from Oxford Instruments Technology China.
The work was supported by National Natural Science Foundation of China, the Key Research Program of the Chinese Academy of Sciences and the National Science and Technology Major Project.