Researchers Reveal Degradation Mechanism of Non-precious Metal Catalysts for Fuel Cells
The development of efficient non-precious metal catalysts (NPMCs) can help reduce the cost of fuel cells and accelerate their commercialization.
At present, non-precious metal catalysts suffer from poor stability in proton exchange membrane fuel cells, and their degradation mechanism at the molecular scale is unclear.
Recently, a research group led by Prof. SUN Gongquan Sun and Prof. WANG Suli collaborated with Prof. WANG Junhu's group from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) revealed the degradation mechanism of Fe-N-C-type NPMCs.
This work was published in Applied Catalysis B: Environmental on March 4.
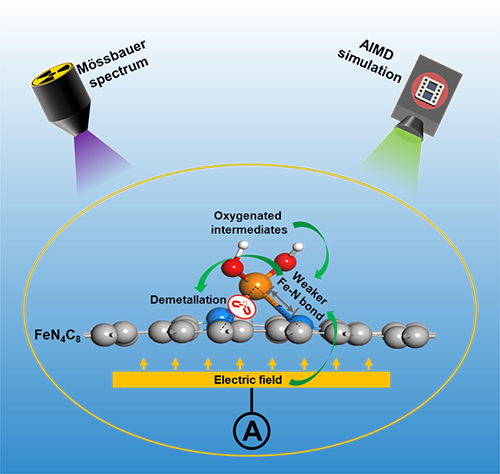
The influence of structure and structural evolution of active site on the demetallation of Fe-N-C is investigated by the end-of-test/in-situ M?ssbauer spectroscopy and ab initio molecular dynamics simulation (Image by XU Xinlong)
The researchers found that D1 was the main active site for oxygen reduction reaction and the demetallation was responsible for the degradation of Fe-N-C. The degradation was initially rapid but slow afterward, which could be explained by the different activity and stability of various FeN4 sites.
Moreover, they revealed that the adsorption of oxygenated intermediate and the motivation of electric field could further weaken the Fe-N bond in FeN4, leading to more severe demetallation. The faster degradation of Fe-N-C under higher potential was due to the stronger adsorption and field intensity.
This work provides insight into the degradation mechanism of Fe-N-C at the molecular level. It can work as the guidance for the future development of stable NPMCs in fuel cell devices.
This work was supported by the National Natural Science Foundation of China, and the Postdoctoral Science Foundation of China. (Text by XU Xinlong)