Synergistic Effect of Surface Hydride Species and Ru Clusters Improves Efficiency of Ammonia Synthesis
A research team led by Prof. CHEN Ping and Associate Prof. LIU Lin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Associate Prof. WU Anan from Xiamen University, discovered that samarium hydride (Sm-H) species, in situ generated on the surface of Ru clusters/Sm2O3 catalyst under reaction conditions, could work synergistically to enhance the catalytic activity of Ru clusters for ammonia synthesis.
This study was published in ACS catalysison Jan. 28.
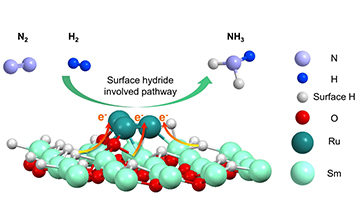
Schematic illustrations of the synergistic effect of surface hydride species and Ru clusters for catalytic ammonia synthesis reaction (Image by ZHANG Xilun)
The researchers synthesized sub-nanometer Ru clusters on Sm2O3 (Ru clusters/Sm2O3) and investigated its catalytic performance for ammonia synthesis. "Under mild reaction conditions, this catalyst showed good activity as well as high stability, thermal stability, and water-tolerant stability," said Prof. CHEN.
Furthermore, experimental and theoretical results revealed that Sm2O3 featuring surface Sm-H species could regulate the electronic structure of Ru clusters, making the catalyst active for hydrogen and nitrogen activation. And the Sm-H species on the Ru/Sm2O3 catalyst could directly participate in the formation of ammonia, rendering the catalyst exceptionally active in ammonia synthesis.
"The discovery of the synergy role of surface hydride species can deepen the molecular-level understanding of active sites and reaction mechanism of Ru-based catalysts during the ammonia synthesis process," said Prof. CHEN, "and it also provides an opportunity for the rational design of efficient Ru-based ammonia synthesis catalysts under mild conditions."
This work was supported by the National Natural Science Foundation of China, Dalian National Laboratory for Clean Energy, and K. C. Wong Education Foundation. (Text by ZHANG Xilun and LIU Lin)