Photoredox Catalysis Realizes Halopyridylation of Alkenes
A research team led by Prof. CHEN Qing-An from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) realized photo-induced catalytic halopyridylation of alkenes using halopyridines and non-activated olefins as simple reaction substrates.
Their findings were published in Nature Communications on Nov. 11.
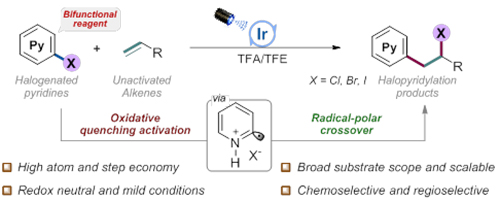
Cross-coupling of aryl halides with alkenes (Image by GUO Shiyu)
The researchers added trifluoroacetic acid to promote the protonation of halopyridines, after which the iridium photocatalyst was more likely to undergo oxidative quenching pathway, and the protonated halopyridines was excited to generate electrophilic pyridyl radicals.
Therefore, the pyridyl radicals could further undergo radical addition with electron-rich non-activated olefins to generate alkyl radical intermediates.
They also found that the iridium photocatalyst in the oxidation state could oxidize the generated alkyl radical to carbocations, and further capture the halide ions in the system to construct new C-C and C-X bonds (X=Cl, Br, I).
This study was supported by the National Natural Science Foundation of China and Liaoning Province Doctoral Research Startup Fund Project. (Text by GUO Shiyu)