"Chainmail Catalysis" Improves Efficiency of CO Oxidation at Room Temperature
CO oxidation at room temperature is significant for gas purification. Pt promoted by 3d transition metals (TMs) is a promising candidate for this reaction. However, TMs are prone to be deeply oxidized in an oxygen-rich atmosphere, leading to low activity.
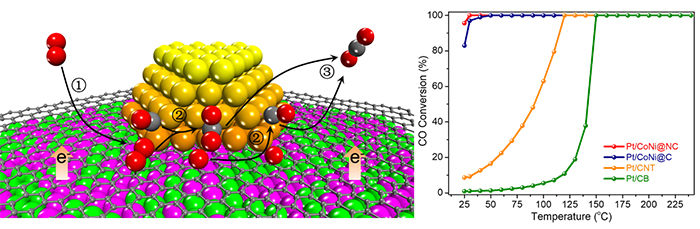
Recently, a research group led by Prof. DENG Dehui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) designed a chainmail catalysis of graphene-isolated Pt from CoNi nanoparticles (Pt|CoNi) for CO oxidation at room temperature.
The study was published in Nature Communication on October 04.
Graphene-isolated Pt from CoNi nanoparticles (Pt|CoNi) for efficiently catalytic CO oxidation (Image by HU Jingting)
CoNi alloy was protected by ultrathin graphene shell from oxidation and therefore modulated the electronic property of Pt-graphene interface via electron penetration effect. It achieved near 100% CO conversion at room temperature, while there were limited conversions over Pt/C and Pt/CoNiOx catalysts.
By experiments and theoretical calculations, the researchers indicated that CO could saturate Pt sites, but O2 could adsorb at the Pt-graphene interface without competing with CO, which facilitated the O2 activation and the subsequent surface reaction.
"The graphene-isolated system in this work is distinct from the classical metal-metal oxide interface for catalysis, and it provides a new thought for the design of heterogeneous catalysts," said Prof. DENG.
This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the Strategic Priority Research Program of CAS, and Collaborative Innovation Center of Chemistry for Energy Materials (2011. iChEM). (Text by HU Jingting)