New Strategy Achieved Efficient and Stable Carbon Dioxide Electrolysis in Solid Oxide Electrolysis Cell
Solid oxide electrolysis cell (SOEC) is promising in CO2 conversion and renewable clean electricity energy storage. It can convert CO2 and H2O simultaneously into syngas or hydrocarbon fuel at the cathode, and produce high purity O2 at the anode.
Perovskite-type oxides have the advantages of excellent doping ability, carbon deposition resistance and redox stability. However, the application of perovskite electrodes is limited due to insufficient electrocatalytic activity.
Recently, a research team led by Prof. WANG Guoxiong and Prof. BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) and their collaborators achieved efficient and stable CO2 electrolysis in SOEC. They found that redox cycle manipulations promoted the exsolution of high-density metal/perovskite interfaces, which improved the CO2 electrolysis performance and stability.
This study was published in Nature Communications on September 27.
High-density metal/perovskite interfaces achieve efficient and stable carbon dioxide electrolysis in solid oxide electrolysis cell (Image by LV Houfu and LIN Le)
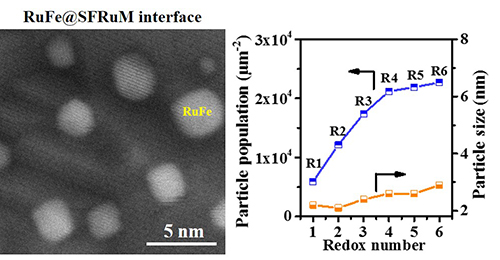
The researchers prepared Ru-doped Sr2Fe1.4Ru0.1Mo0.5O6-δ (SFRuM) double perovskite. They found that the repeated redox manipulations promoted the exsolution of RuFe alloy nanoparticles from 5900 μm-2 to 22680 μm-2, where the mean particle size was between 2.2 and 2.9 nm, thus regulating the density of the density of the RuFe@SFRuM interfaces.
Combined with in situ atmosphere electron microscopy, elemental maps and electron energy loss spectroscopy, they revealed the formation and regeneration mechanism of RuFe@SFRuM interface under reducing and oxidizing atmosphere. "The enrichment in Ru species on the surface can promote the exsolution of high-density RuFe@SFRuM interfaces," said Prof. WANG.
What's more, in situ atmosphere electron microscopy, electrochemical impedance spectroscopy combined with density functional theory calculations confirmed that the RuFe@SFRuM interface promoted CO2 adsorption and activation. Compared with the SFRuM cathode, the RuFe@SFRuM cathode had a 74.6% increase in current density for CO2 electrolysis at 1.2 V and 800 °C, and exhibited a high stability of CO2 electrolysis for 1000 h.
The above work was supported by the National Natural Science Foundation of China, the National Key Research and Development Program, and the Youth Innovation Promotion Association of the Chinese Academy of Sciences. (Text/Picture Houfu Lv and Le Lin)