Study Unveils Dynamic Behavior of Cu-N-C Single-Atom Catalysts in Electrocatalysis
Atomically dispersed M-N-C (M refers to transition metals) materials are regarded as the most promising alternatives to the Pt-based precious-metal catalysts for the electrochemical reduction of oxygen (ORR). However, the genuine active sites in M-N-C still remain elusive.
Recently, Prof. ZHANG Tao's, Prof. WANG Aiqin's and Prof. YANG Xiaofeng's group from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. LI Jianfeng and Prof. TIAN Zhongqun from Xiamen University, synthetized a uniform and well-defined Cu-N-C single-atom catalyst (Cu-N-C SAC), and unveiled the dynamic behavior of Cu-N-C SAC during the ORR process.
This study was published in the Journal of American Chemical Society on August 31.
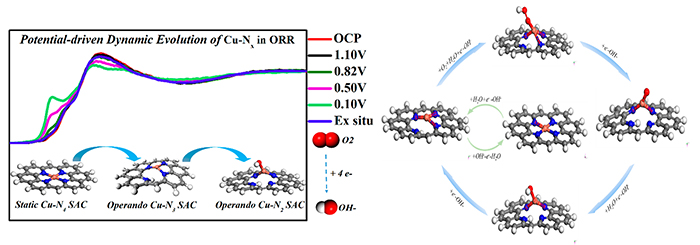
The operando XANES and proposed catalytic mechanism for the alkaline ORR on the Cu-N-C SAC (Image by YANG Ji)
Local coordination structure of SAC plays an important role in catalytic performance. The active site structure in SACs will undergo dynamic changes during the reaction.
The researchers prepared a uniform and well-defined Cu-N4 SAC that exhibited a comparable alkaline ORR activity to Pt/C. They found that the as-prepared Cu-N4 structure in Cu-N4 was firstly transformed to Cu-N3 structure driven by the applied potential, and then to HO-Cu-N2 structure under reaction conditions.
"These results provide a new vision to understand the reaction mechanism of M-N-C SACs as well as a guide to the rational design of more active SACs," said Prof. WANG Aiqin.
The above work was supports by the National Natural Science Foundation of China, the Strategic Priority Research Program of the CAS, the Dalian National Laboratory for Clean Energy (DNL) Cooperation Fund of CAS, and the BL14W at Shanghai Synchrotron Radiation Facility (SSRF). (Text by YANG Ji)