Biomimetic Approach to Achieve Catalytic Enantioselective Synthesis of Tetracyclic Isochroman
Tetracyclic isochromans, a type of polyketide oligomers, are ubiquitously present in numerous natural products and bioactive molecules.
Recently, a group led by Prof. LI Can and Prof. LIU Yan from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) prepared a diversity of tetracyclic isochromans with high levels of diastereoselectivities and enantioselectivities by biomimetic approach.
This study was published in nature communications on August 16.
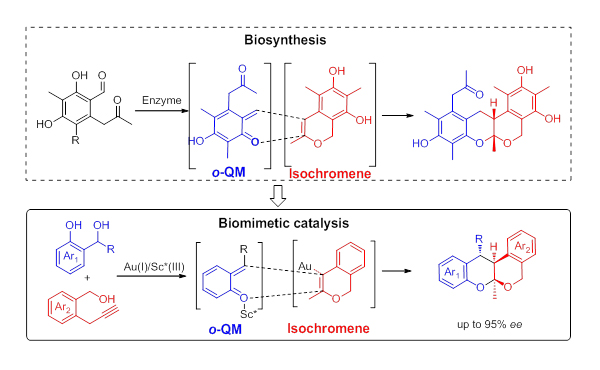
Biomimetic approach to the catalytic enantioselective synthesis of tetracyclic isochroman (Image by LIN Xiangfeng and LIU Yan)
Ortho-quinone methide (o-QM) is proposed to be the diene intermediate in the biosynthesis of several polyketide natural products via hetero-Diels-Alder (HDA) reaction.
The researchers designed a highly efficient Au(I)/chiral Sc(III) bimetallic catalytic system by mimicking the biosynthetic pathway of polyketide oligomer natural products, and achieved asymmetric HDA reaction of in-situ generated isochromene and ortho-quinonemethide (o-QM).
They prepared a series of chiral tetracyclic isochromans from readily available -propargyl benzyl alcohols and 2-(hydroxylmethyl) phenols under mild conditions.
The study demonstrated the potential utility of biomimetic synthesis in the development of reaction and expanding the limitation of substrates. It was supported by the National Natural Science Foundation of China.(Text by LIN Xiangfeng and LIU Yan)