Novel Iron-based Catalysts Boosts Conversion of CO2 to Higher Alcohols
Higher alcohols (C2+OH), important intermediates for fine chemicals, are mainly produced via petrochemical route, which is energy-intensive and environmentally unfriendly.
Recently, a research team led by Prof. SUN Jian and Prof. GE Qingjie from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) proposed a monometallic iron catalyst with Na and S co-modification for higher alcohols synthesis from CO2 hydrogenation.
This study was published in Applied Catalysis B: Environmental on July 28.
Iron is a well-known candidate catalyst for CO2 conversion. However, the strong ability for CO dissociation of the monometallic iron catalyst decreases the efficiency of higher alcohols synthesis.
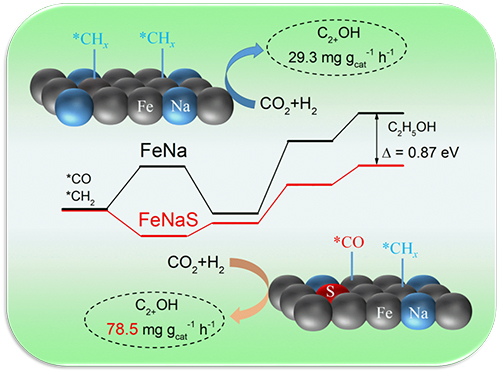
Revealing the promoting role of S in higher alcohols synthesis over iron catalysts (Image by YAO Ruwei)
The proposed monometallic iron catalyst achieved a space-time yield of 78.5 mg gcat-1 h-1 for C2+ alcohols at a relatively mild condition, which was comparable to the composite catalysts such as FeCu and FeRh.
The synergistic effects of Na and S enabled the Fe sites in different electronic environment in one metal phase and helped provide matched dissociative and non-dissociative CO activation simultaneously required for higher alcohols synthesis.
"This work extends the understanding of the role of sulfur for iron catalysts and points to a new and general direction for catalyst design in higher alcohols synthesis," said Prof. SUN.
This work was supported by the National Natural Science Foundation of China, Strategic Priority Research Program of the Chinese Academy of Sciences, and Liaoning Revitalization Talents Program. (Text by YAO Ruwei)