Scientists Unveil Potential Dependence in Nitric Oxide Electroreduction to Ammonia
Nitrogen oxide (NOx), such as nitric oxide (NO), are environmental pollutants. They are often removed via selective catalytic reduction (SCR) technology.
A novel artificial nitrogen cycle path driven by electrocatalysis has been proposed to couple conventional denitrification and ammonia (NH3) synthesis. However, further studied showed that direct electroreduction of NOx to N2 was difficult in any potential.
Recently, a research group led by Prof. XIAO Jianping from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences unveiled the potential dependence of products selectivity in electrochemical NO reduction (eNORR) to ammonia.
This study was published in The Journal of Physical Chemistry Letters on July 20.
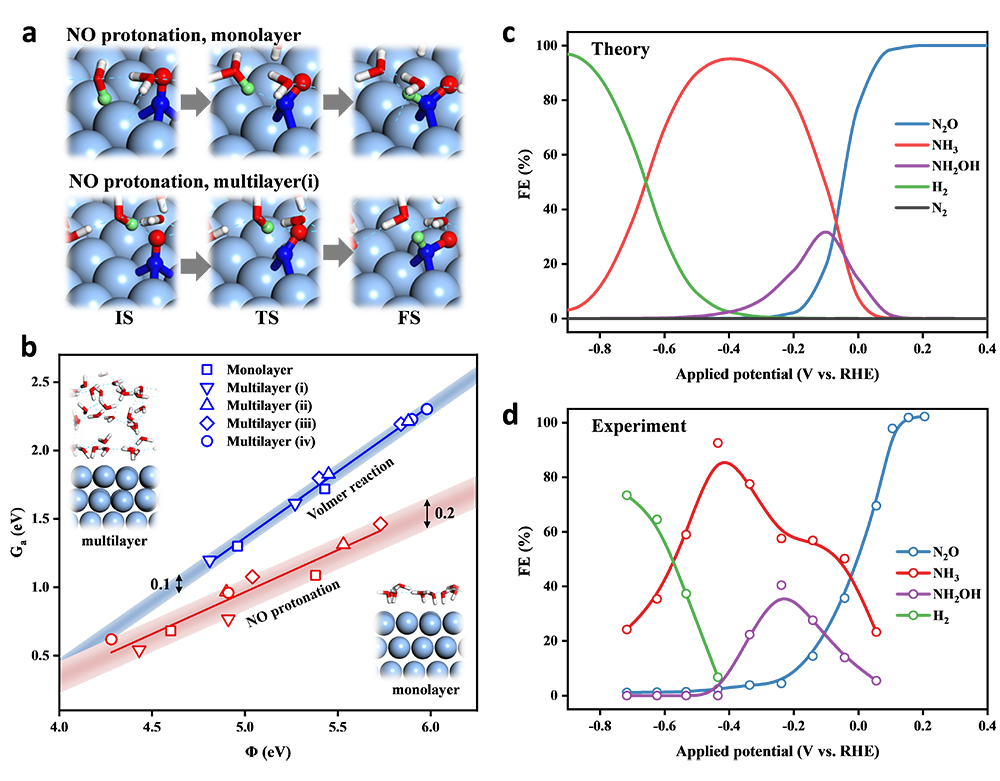
(a)-(b) Verification of monolayer water model in electrocatalytic energy barrier calculations; (c)-(d) Comparison of theoretical and experimental Faradaic efficiency (Image by LONG Jun)
The researchers took Ag as model catalyst. They verified the reliability of monolayer water model in electrocatalytic energy barrier calculations and obtained the energetics of NORR network by density functional theory calculations. Finally, they developed a microkinetic model to rationalize the general selectivity trend of eNORR with varying potential.
This model reproduced the experimental Faradaic efficiency well, quantitatively describing the selectivity turnover from N2O to NH3 and from NH3 to H2 as applies more negative potential.
The first turnover of selectivity was due to the thermochemical coupling of two NO* limiting the N2O production. The second turnover was attributed to the more significant potential-dependence of HER than NH3 production.
"This model provides a theoretical guide for the design of selective electrocatalytic of NOx, which is also beneficial to understand the potential dependence of some other electrocatalytic reduction reactions," said Prof. XIAO.
The above work was supported by the DNL Cooperation Fund of CAS, the National Natural Science Foundation of China, and the Strategic Priority Research Program of CAS. (Text by LONG Jun)