Study Reveals Regulation Mechanism of Integrin α4β7 Mediated HIV-1 Virus Infection
Recently, a research group led by Prof. LI Guohui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. CHEN Jianfeng's group from Shanghai Institute of Biochemistry and Cell Biology of CAS, revealed the regulation mechanism of integrin α4β7 mediated human immunodeficiency virus 1 (HIV-1) infection.
This study was published in Signal Transduction and Targeted Therapy on July 16.
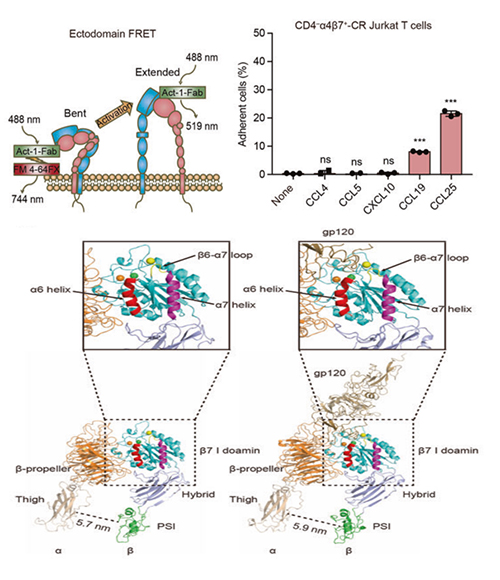
Experiment setup for measuring FRET efficiency between integrin α4β7 βI domain and the plasma membrane (top left); Adhesion of integrin α4β7 T cells to the immobilized gp120 with different chemokine stimulations (top right); Molecular details underlying the conformational rearrangements integrin α4β7 headpiece, which direct correlations with integrin activation and gp120 binding (bottom) (Image by ZHANG Yuebin and LI Guohui)
Integrin α4β7, an important cell surface adhesion molecule, is responsible for mediating lymphocytes from blood circulation into the intestine and central nervous system. The abnormality of its function is closely related to human autoimmune diseases.
Previous studies have shown that intestinal homing CD4+ T cells expressing integrin α4β7 are the early targets of HIV-1 virus infection, which plays an important role in the pathogenesis of HIV-1 infection.
The binding between the envelope protein gp120 located on the surface of the HIV-1 virus and the receptors on the surface of CD4+ T cells is a key step for HIV-1 to infect T cells.
In this study, the researchers investigated the interaction between integrin α4β7 and gp120. They found that specific intestinal chemokines could stimulate integrin α4β7 in a relatively stretched condition, leading to a highly activated conformation state, and it enabled integrin α4β7 to bind the HIV-1 envelope protein gp120. While, the inactive integrin α4β7 exhibited no binding ability with HIV-1 envelope protein gp120.
Moreover, they indicated that the interactions between the metal ion-dependent adhesion site (MIDAS) in the integrin β7 subunit and the highly conserved tripeptide LDI in the HIV-1 envelope protein gp120 were the key site for integrin α4β7 mediated HIV-1 infection.
In addition, they also found that the interactions between integrin α4β7 and HIV-1 envelope protein gp120 might activate multiple intracellular signaling pathways, which further regulated HIV-1 virus replication and T cell function.
"This study would provide new strategies and ideas for the prevention and treatment of HIV-1 infection and the screening of related drugs," said Prof. LI.
The research was supported by the National Natural Science Foundation of China. (Text by ZHANG Yuebin and LI Guohui)