Newly Developed Ion-conducting Membrane Improves Performance of Alkaline-zinc Iron Flow Battery
Alkaline zinc-iron flow battery (AZIFB) is well suitable for stationary energy storage applications due to its advantages of high open-cell voltage, low cost, and environmental friendliness. However, it surfers from zinc dendrite/accumulation and relatively low operation current density.
Recently, a research group led by Prof. LI Xianfeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Science (CAS) developed layered double hydroxide (LDH) membrane with high hydroxide conductivity and ion selectivity for alkaline-zinc iron flow battery.
The study was published in Nature Communications on June 7.
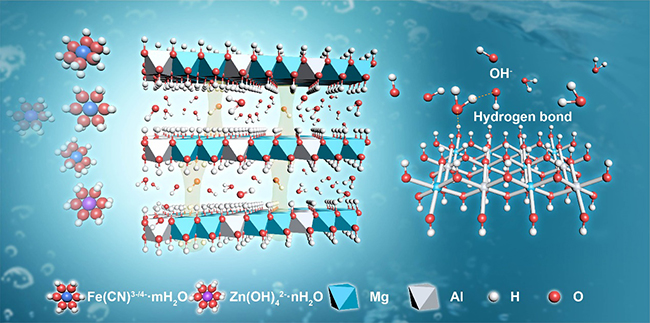
Selective ions transport and the hydroxide ions transport in LDHs (Image by HU Jing)
In order to enhance the operating current density of AZIFB, the researchers added LDHs nano materials into the AZIFB and designed a LDHs-based composite membrane with high performance. High selectivity and superb hydroxide ion conductivity were achieved through the combination of the well-defined interlayer gallery with a strong hydrogen bond network along 2D surfaces.
They identified that surface -OH groups of LDHs layer could assist the conduction of OH- by promoting proton transfer away from one water molecule to the original OH-.
Because of the high ionic conductivity, the LDHs-based membrane enabled the AZIFB to operate at 200 mA cm-2, along with an energy efficiency of 82.36%.
"This study offers a new insight to design and manufacture high-performance membranes for AZIFB," said Prof. LI.(Text by YUAN Zhizhang)