Researchers Quantify Thermodynamic Interplay during Protein Co-aggregation
Co-aggregation of multiple pathogenic proteins is common in neurodegenerative diseases. However, the deconvolution of such biochemical process is still challenging.
Recently, a research group led by Professor LIU Yu from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences developed a dual-color fluorogenic thermal shift assay to simultaneously demonstrate the aggregation of two different proteins and quantitatively study their thermodynamic stability during co-aggregation.
This study was published in Chemical Science on May 20.
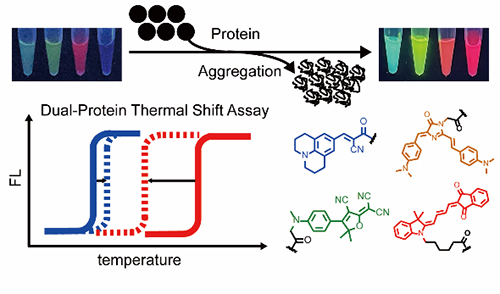
A series of multi-color protein aggregation sensors and a dual-color thermal shift assay to report on protein co-aggregation of two different proteins simultaneously and quantitatively (Image by BAI Yulong)
The researchers developed multi-color fluorogenic protein aggregation sensors to expand spectral coverage. Then they quantified shifts in melting temperatures in a heterozygous model protein system, which revealed that the thermodynamic stability of wild-type proteins was significantly compromised by the mutant ones but not vice versa.
They also examined how small molecule ligands selectively and differentially interfered with such interplay.
"These sensors are suited to visualize how different proteins exert influence on each other upon their co-aggregation in live cells," said Prof. LIU.
In particular, they investigated how amyloidogenic transthyretin proteins interact with apolipoprotein IV proteins during their co-aggregation process, which indicated the apolipoprotein IV was a pharmacological chaperone.
This work was support by National Natural Science Foundation of China, the Liaoning Revitalization Talents Program, China Postdoctoral Science Foundation Grant, and Pfizer ASPIRE award for transthyretin amyloidosis basic research. (Text by BAI Yulong)