New Single-atom Catalysis Boots Reductive Amination Reaction
The geometric isolation of metal species in single-atom catalysis (SACs) not only maximizes the atomic utilization efficiency, but also endows SACs with unique selectivity in various transformations.
The coordination environment of isolated metal atoms in SACs determines the catalytic performance. However, it remains challenging to modulate the coordinative structure while still maintain the single-atom dispersion.
Recently, a research group led by Prof. ZHANG Tao and Prof. WANG Aiqin from the Dalian Institute of Chemical Physics of the Chinese Academy of Sciences fabricated Ru1/NC SAC with good catalytic activity, selectivity and stability in reductive amination of biomass derived aldehydes/ketones to produce primary amines, and elucidated the structure-performance relationships from the atomic/molecular level.
This study was published in Nature Communications on June 2.
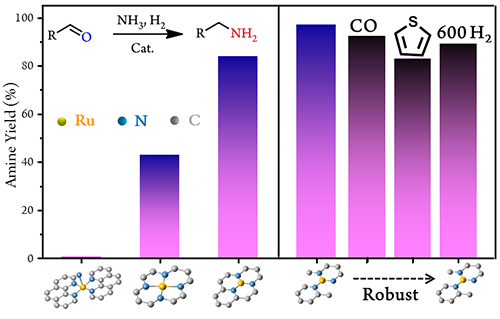
Highly selective and robust single-atom catalyst R1/NC for reductive amination of aldehydes/ketones (Image by QI Haifeng)
The researchers prepared Ru1/NC SAC towards the target reaction, and discovered both catalytic activity and selectivity increased with the decrease of Ru-N coordination numbers.
Particularly, Ru1/NC SAC with Ru1-N3 moiety offered the best catalytic performances, which was much superior to the ever reported nanoparticulate and homogeneous Ru catalysts.
Moreover, Ru1/NC presented excellent durability against poisoning by CO or sulfur-containing compounds, and the single-atom dispersion was well maintained even after reduction under extreme conditions.
This work was supported by the National Key Projects for Fundamental Research and Development of China, the National Natural Science Foundation of China, and the Strategic Priority Research Program of the CAS. (Text by QI Haifeng)