Metallic State of Ag Nanoclusters in Oxidative Dispersion Identified in situ
Oxidative dispersion has been widely used in the regeneration of sintered metal catalysts as well as the fabrication of single-atom catalysts.
The consensus on the oxidative dispersion process includes the formation of mobile metal oxide species from large metal particles and the capture of these species on a support surface. Nevertheless, the mechanism of oxidation-induced dispersion has yet to be confirmed via in situ electron microscopic and/or spectroscopic characterizations.
Recently, a research team led by Prof. FU Qiang and Prof. BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. YANG Bing from DICP and Prof. GAO Yi from the Shanghai Institute of Applied Physics of CAS, reported the oxygen adsorption-induced dispersion of metallic Ag nanoclusters in a typical oxidative atmosphere.
The results were published in Nature Communications on March 3.
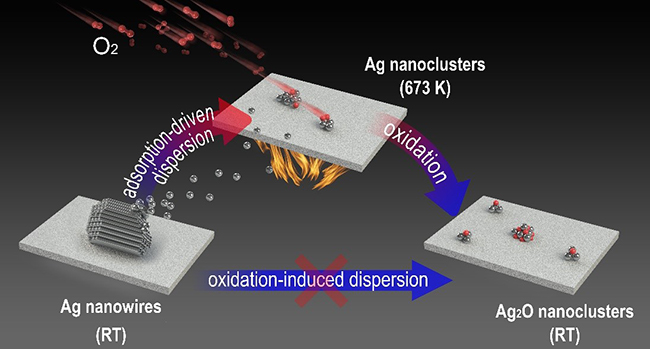
Dynamic evolution of Ag nanostructures during oxidative dispersion (Image by LI Rongtan)
By utilizing in situ imaging methods such as environmental scanning electron microscopy (ESEM), and newly developed near-ambient pressure photoemission electron microscopy (NAP-PEEM), researchers found that micron-scale Ag nanowires could be dispersed into subnanometer clusters under an oxygen atmosphere.
Ex situ experiments indicated that Ag nanowires were converted into AgOx nanoclusters. Conversely, in situ near-ambient pressure photoelectron spectroscopy (NAP-XPS) directly demonstrated the presence of a transitional state of metallic Ag nanoclusters during dispersion at high temperatures, while the formation of the oxide occurred during the cooling process. The dynamic dispersion of Ag nanowires during CO oxidation was also demonstrated.
Based on experimental and theoretical calculations, chemisorption of oxygen from the O2 atmosphere was shown to be the essential driving force for the dispersion of metallic Ag nanoclusters.
This work provides a new understanding of the role of the O2 atmosphere in oxidative dispersion, which is particularly important for the prediction and control of the dynamic dispersion/redispersion of supported metal catalysts under similar reaction conditions.
This work was supported by the National Natural Science Foundation of China, the Strategic Priority Research Program (B) of the Chinese Academy of Sciences, the Ministry of Science and Technology of China, and the DNL Cooperation Fund.
Dalian Institute of Chemical Physics, Chinese Academy of Sciences
457 Zhongshan Road, Dalian, 116023, China
Tel: 86-411-84374221
E-mail: wangyj@dicp.ac.cn