Orthogonal Regulation of Nucleophilic and Electrophilic Sites in Pd-Catalyzed Regiodivergent Couplings between Indazoles and Isoprene Developed
Dimethylallyl-related units plays a significant role in enhancing the lipophilicity of molecules and facilitating permeation across the cellular membrane.
Isoprene can serve as an attractive precursor in the chemical synthesis of dimethylallyl-derived molecules in an atom-economic pathway, owing to its low cost and large-scale production. And indazoles are pharmacologically important scaffolds that found in numerous natural products and drugs.
Recently, a team led by Prof. CHEN Qing'an from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in cooperation with Associate Prof. JIANG Xuliang from Shenyang Pharmaceutical University, developed an orthogonal regulation of nucleophilic and electrophilic sites in Pd-catalyzed regiodivergent couplings between indazoles and isoprene.
Their findings were published in Angew. Chem. Int. Ed. on Jan. 18.
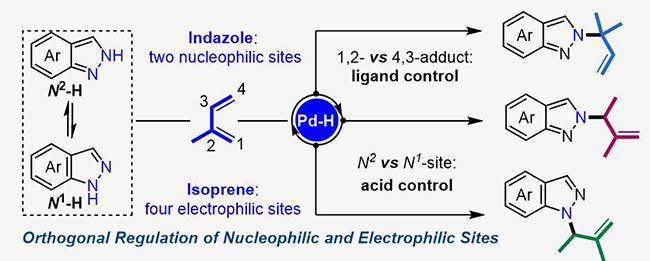
Pd-catalytic regiodivergent dimethylallylation of indazole with isoprene (Image by JIANG Wenshuang and JI Dingwei)
The researchers found that the 1,2-or 4,3-insertion pathway with respect to the electrophilic sites on isoprene could be controlled by the choice of ligands under Pd-hydride catalysis. In terms of the nucleophilic sites on indazoles, the reaction occured at either the N1-or N2-position of indazoles was governed by the acid co-catalysts.
This orthogonal regulation strategy of nucleophilic and electrophilic sites offers new opportunities for the buildup of molecular complexity.
This study was supported by the National Natural Science Foundation of China and Liaoning Revitalization Talents Program.
Dalian Institute of Chemical Physics, Chinese Academy of Sciences
457 Zhongshan Road, Dalian, 116023, China
Tel: 86-411-84374221
E-mail: wangyj@dicp.ac.cn