Researchers Proposed "Reaction Phase Diagram" for Computational Design of Chemical Reactions
Chemical reactions on solid catalyst surfaces are complicated with high-dimensional variables. A better way towards the catalyst design is the establishment of activity and selectivity trends over a collection of catalysts.
It was found that there are scaling relations between different adsorption energies and Bronsted–Evans–Polanyi (BEP) relations between activation barriers and relevant reaction energies, which in principle make it feasible to establish activity trends by a descriptor.
The traditional design scheme can be summarized as Scaling relation → BEP relation → Activity trend → Catalyst design. However, there are still two limits. On one hand, it was usually based on pre-assumed reaction pathways. In addition, it is also difficult to solve microkinetic models as there are thousands of reaction pathways.
Accordingly, it is still highly risky of designing a new catalytic reaction prior to experimental studies.
Recently, a group led by Prof. XIAO jianping from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) reviewed an innovate scheme, namely reaction phase diagram (RPD), which can offer not only an in‐depth understanding of reaction mechanisms, but also the prediction of catalytic activity and selectivity trend over a collection of catalysts.
This study was published in WIREs Comput. Mol. Sci. on Jan. 5.
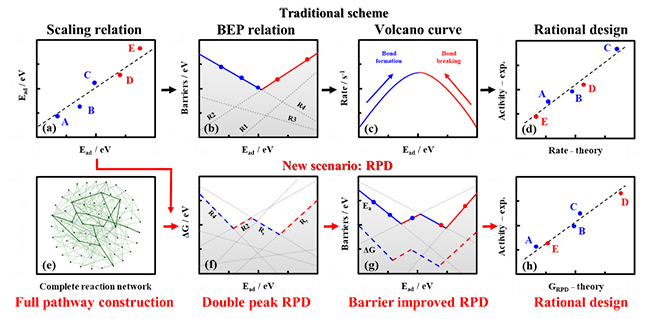
Figure: Illustration of traditional strategy (black arrow) and innovate reaction phase diagram (red arrow) for catalyst design (Image by GUO Chenxi)
The theoretical catalyst design of RPD started from a "full pathway construction", where all possible reaction pathways could be enumerated based on a set of elementary steps. Different optimal mechanisms could be obtained following the global optimization considering all possible pathways at given descriptors, and the activity optimums could be derived due to diverse reaction pathways.
"We could obtain more accurate activity trends based on the GRPD-limiting energies with kinetic calculations, and it could result in more reasonable analysis of mechanism and catalyst design," said Prof. XIAO.
The RPD analysis with the scheme of reaction global optimizations was applied to understand various catalytic processes, including the double-peak activity trend for CO2 electroreduction, the potential dependent activity trend for oxygen reduction reaction (ORR), and the complex selectivity analysis in CO conversion producing C2 products. Moreover, it provided a successful case of catalyst rational design with a target of NO selective electroreduction to ammonia.
This study was supported by the National Natural Science Foundation of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, and the Liaoning Revitalization Talents Program.
Dalian Institute of Chemical Physics, Chinese Academy of Sciences
457 Zhongshan Road, Dalian, 116023, China
Tel: 86-411-84374221
E-mail: wangyj@dicp.ac.cn