Researchers Achieve High-efficient Conversion of Methane to Formic Acid under Mild Conditions
Methane is promising energy resources for producing high-value-added chemicals. Methane conversion to value-added chemicals or fuels under mild conditions has become one of the hottest topics in energy and catalysis with the need of developing more economical and environmental-friendly path in contrast to the energy-intensive industrial process.
However, the high symmetry and low polarizability of methane molecule render it highly challenging to activate methane under mild conditions. In addition, the target products are usually more reactive than the methane, which are subject to over-oxidation to the greenhouse gas CO2.
Recently, a group led by Prof. DENG Dehui and Assoc. Prof. YU Liang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) reported highly-dispersed coordinatively unsaturated Fe-sites confined within the ZSM-5 channels enabled highly efficient methane conversion to formic acid (HCOOH) under mild conditions.
This work was published in Nano Energy on Dec. 29 in 2020.
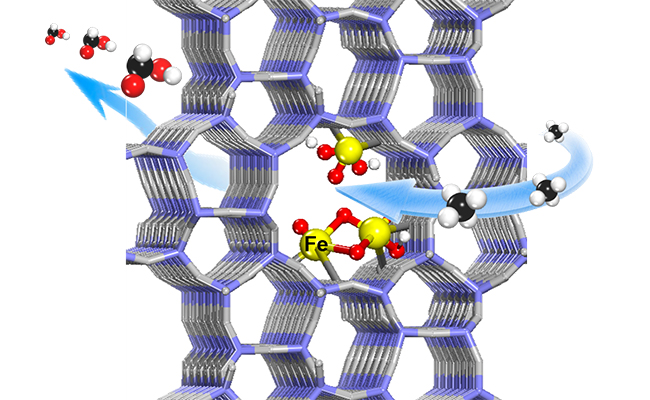
Methane conversion to formic acid on the atomically-dispersed Fe sites confined within the channels of ZSM-5 (Image by (Text by ZHU Kaixin and GAO Hehua)
The researchers reported that highly efficient and selective oxidation of methane to HCOOH could be achieved on the atomically-dispersed Fe sites confined within the channels of ZSM-5. Via adjusting the silica-alumina ratio and Fe loading in the ZSM-5 to modulate the microenvironment of the active Fe species, they reached a turnover frequency (TOF) of 84200 h-1 for producing C1 oxygenates and a high HCOOH selectivity of 91% with productivity of 383.2 mmol gcat.-1 h-1 at 80 °C
"This result surpassed all previously reported methane conversion catalysts operated under similar conditions," said Prof. DENG.
Furthermore, by combining various characterizations and density functional theory calculations, they found that Fe-O active sites could be generated from H2O2 dissociation on both the mononuclear and binuclear Fe centers confined within the channels of ZSM-5.
The Fe-O active sites could facilitate the activation and cleavage of the C-H bond of methane and thereby promoted successive oxidation of methane to formic acid through methanol and formaldehyde via free radical pathways, while inhibiting the over-oxidation to CO2.
This study paves a new route to design and construct lattice-confined active sites for highly efficient conversion of methane under mild conditions.
This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the Strategic Priority Research Program of CAS, the DNL Cooperation Fund, CAS.
Dalian Institute of Chemical Physics, Chinese Academy of Sciences
457 Zhongshan Road, Dalian, 116023, China
Tel: 86-411-84374221
E-mail: wangyj@dicp.ac.cn