Photopolymerized Gel Electrolyte with High Room Temperature Ionic Conductivity for Solid-state Sodium Metal Batteries Developed
The growing demand for lithium-ion batteries (LIBs) has sparked fears of a potential Li shortage. In order to achieve long-term development goal, low-cost alternative energy storage technologies are urgently needed.
Sodium (Na) element has similar properties to lithium (Li), but is much more abundant and widely distributed, which make sodium-ion batteries (SIBs) a highly competitive alternative to LIBs. However, the ionic radius of Na+ is larger than Li+, which is not suitable for the intercalation reaction of conventionally used graphite anode in LIBs.
Na metal, with high theoretical capacity and a low redox potentiais regarded as an ideal anode material of SIBs with high output voltage and energy density. Despite these advantageous features, sodium metal batteries (SMBs) in organic electrolyte systems still suffer from serious safety issues, such as the leakage of electrolyte, and dendrite formation of Na, which substantially inhibit the applicability of this type of battery.
Recently, a research team led by WU Zhong-Shuai from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences developed a photopolymerized gel electrolyte with high room temperature ionic conductivity, wide electrochemical window and excellent flexibility for ultra-high rate and ultra-long cycling sodium metal battery.
This work was published in Advanced Energy Materials on Nov. 10.
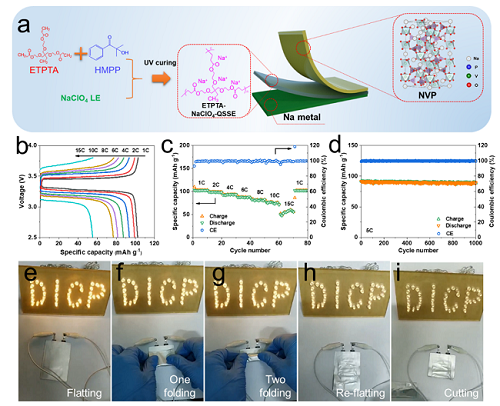
Photopolymerized gel electrolyte with unprecedented room‐temperature ionic conductivity for high‐energy‐density solid‐state sodium metal batteries was developed (Image by WEN pengchao and HOU Xiaocheng)
"We prepared a novel high ionic conductivity polymer, ethoxylated trimethylolpropane triacrylate (ETPTA) based quasi-solid-state electrolyte (ETPTA-NaClO4-QSSE) by photopolymerization for high-energy-density solid-state SMBs," said Prof. WU.
The resulting ETPTA-NaClO4-QSSE exhibited unprecedented room-temperature ionic conductivity of 1.2 mS/cm, and provided a wide electrochemical window up to 4.7 V (vs. Na+/Na) and extraordinary interfacial compatibility with Na metal anodes, effectively inhibiting the growth of Na dendrites.
Moreover, the as-assembled solid-state SMBs (NVP||ETPTA-NsaClO4-QSSE||Na) offered high specific capacity of 101 mAh/g at 1 C, and record rate performance at room temperature.
Furthermore, the full battery of NVP||ETPTA-NaClO4-QSSE||Na in pouch cell showed excellent flexibility and safety, demonstrative of wide applicability.
This work was supported by the National Natural Science Foundation of China, National Key R&D Program of China, Natural Science Foundation of Liaoning Province, Dalian National Laboratory For Clean Energy (DNL), CAS.
Dalian Institute of Chemical Physics, Chinese Academy of Sciences
457 Zhongshan Road, Dalian, 116023, China
Tel: 86-411-84374221
E-mail: wangyj@dicp.ac.cn