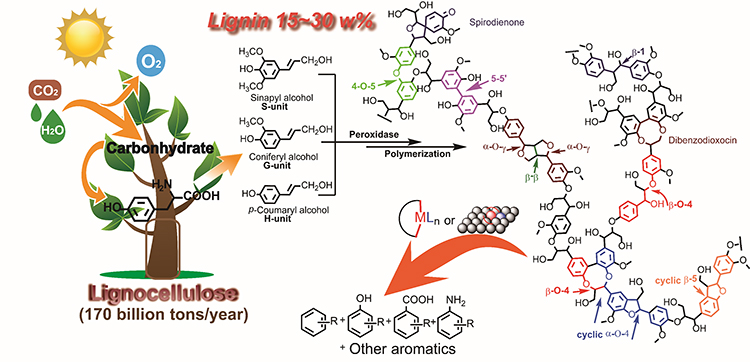
Catalytic Lignin Depolymerization to Aromatic Chemicals. (Image by ZHANG Chaofeng)
In recent decades, research on lignin depolymerization and its downstream product transformation has drawn an enormous amount of attention from academia to industry worldwide, aiming at harvesting aromatic compounds from this abundant and renewable biomass resource.
Although the lignin conversion can be traced back to the 1930s and various noncatalytic and catalytic methods have been explored to depolymerize lignin via direct lignin conversion research or lignin models conversion studies, the complexity of the lignin structure, various linkages, the high stability of lignin bonds, and the diverse fragments condensation process make lignin depolymerization to monomers a highly challenging task.
For the potential practical utilization of lignin, compared with lignin conversion to liquid fuel with extra H2 consumption, maintaining the aromatic structure and preparing high-value aromatic chemicals from renewable lignin is more profitable. Therefore, lignin depolymerization to easy-to-handle aromatic monomers with acceptable conversion and selectivity is of great importance.
Prof. WANG and co-workers present their ten years studies on lignin’s catalytic conversion to aromatic chemicals in this article. First, they introduce the research on protolignin depolymerization via a fragmentation–hydrogenolysis process in alcohol solvents. Then, focusing on the catalytic cleavage of lignin C–C and C–O bonds, they shed light on a recapitulative adjacent functional group modification (AFGM) strategy for the conversion of lignin models. AFGM strategy begins with the adjacent functional group modification of the target C–C or C–O bond to directly decrease the bond dissociation enthalpy (BDE) of targeted bonds or generate new substrate sites to introduce the cleavage reagent for further conversion. Subsequently, on the basis of these two concepts from AFGM, they summarize their strategies on lignin depolymerization, which highlight the effects of lignin structure, catalyst character, and reaction conditions on the efficiency of strategies.
In short, the key point for lignin depolymerization to aromatics is promoting the lignin conversion and restraining the condensation. Compared with the complex research on direct lignin conversion, this bottom-up research approach, beginning with lignin model research, can make the conversion mechanism study clear and provide potential methods for the protolignin/technical lignin conversion.
In addition, one of their perspectives for lignin utilization is that the products from lignin conversion can be used as monomers for artificial polymerization, such as the simple phenol (PhOH) and other potential acid compounds, or that lignin derivative molecules can be used to synthesize high-value synthetic building blocks.
This work was supported by the Strategic Priority Research Program of the CAS, National Natural Science Foundation of China, China Scholarship Council and DICP. (Text ZHANG Chaofeng)