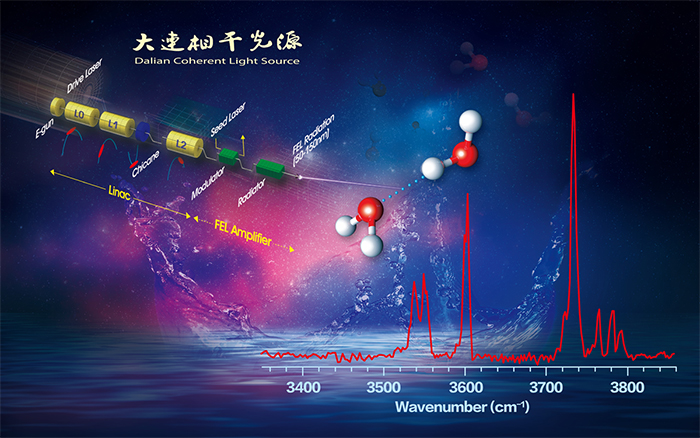
Researchers led by Prof. JIANG Ling, Prof. YANG Xueming and Prof. ZHANG Donghui from Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, collaborated with Prof. LI Jun from Tsinghua University have developed infrared spectroscopy of neutral water dimer based on a tunable vacuum ultraviolet free electron laser. Their studies help to resolve the controversy of the exact vibrational assignment of each band feature of water dimer. The results were published in the journal The Journal of Physical Chemistry Letters.
Clusters consisting of a few to hundreds of atoms exhibit interesting size-dependent properties and are the bridge between molecules and condensed phase bulks. Optical spectroscopy of gas-phase clusters provides detailed structural and dynamical information that is difficult to extract from bulk measurements.
Over the past several decades, enormous efforts were devoted to the spectroscopic study of charged clusters, which allow easy size-selection and detection. In contrast, neutral clusters have presented major experimental challenges, because the absence of a charge makes it difficult for size-selection and detection.
Recently, Dalian Coherent Light Source (DCLS) facility developed by DICP, which delivers VUV Free Electron Laser (FEL) with a continuously tunable wavelength region between 50 and 150 nm and high pulse energy. Inasmuch as clusters with different sizes have different ionization energies, the tunable VUV-FEL light paves the way for selectively ionizing a given neutral cluster free of confinement, thus facilitating realization of their size selectivity. This unique VUV-FEL facility makes it possible to study the IR spectroscopy of confinement-free, neutral clusters via the IR-VUV scheme.
Researchers led by Prof. JIANG Ling and Prof. YANG Xueming from DICP have built an IR-VUV spectroscopy apparatus based on the VUV-FEL and have successfully measured the IR spectra of the water dimer. The water cluster is considered here because of its central role in scientific disciplines ranging from geology to astronomy to biology. The water dimer is a prototype system for the study of the cooperative hydrogen bond (HB) in liquid water, which has proven to be difficult to adequately capture through bulk experiments or theory.
Quantum mechanical calculations on a 12-dimensional ab initio potential energy surface have been utilized to simulate the anharmonic vibrational spectra of the water dimer. The anharmonic vibrational calculations reproduced the well-known four bands of water dimer, which correspond to antisymmetric OH stretch of the proton acceptor, free OH stretch of the proton donor, symmetric OH stretch of the proton acceptor, and hydrogen-bonded OH stretch of the proton donor.
As shown by the bond distances, bond orders, and hybrid orbitals, the O-H bonds in HHOa is stronger than those in the H-ObH due to electron donation from Oa lone pair to the s(OH)* anti-bonding orbitals, the symmetric and antisymmetric OH vibrational frequencies of HHOa are higher than those of H-ObH.
The electronic structure analyses are helpful for understanding the spectroscopic features of OH vibrational modes for different hydrogen bond orientation in more complicated water clusters.
As many clusters have their ionization energies in a range accessible by VUV-FEL light source and near threshold ionization can be readily achieved, the VUV-FEL based IR spectroscopy opens a new paradigm for the study of vibrational spectra of a wide variety of neutral clusters.
The availability of these new experimental data on the neutral clusters is expected to stimulate further calculations and developments of theoretical methods leading to an improved understanding of the structures and dynamics of these systems.
Infrared spectroscopy of neutral water dimer based on a tunable vacuum ultraviolet free electron laser. (Image by JIANG Ling)
This work was supported by the National Natural Science Foundation of China (Grant Nos. 21688102, 21673231, and 91645203), the Strategic Priority Research Program of Chinese Academy of Sciences (CAS) (Grant No. XDB17000000), Dalian Institute of Chemical Physics (Grant No. DICP DCLS201702). (Text by JIANG Ling )