Pressure Makes Best Cooling
Recently, an international research team led by Prof. LI Bing from the Institute of Metal Research, Chinese Academy of Sciences has found that a class of disordered materials, called plastic crystals, exhibit record-large barocaloric effects under very weak pressure. The typical entropy changes are about several hundred joule per kilogram per kelvin, which is ten times better than the previous materials.
Accessing large-scale facilities in Japan (J-PARC and SPring-8) and Australia (ANSTO) to utilize neutron scattering and synchrotron X-ray diffraction techniques, the team revealed that the constituent molecules of these materials are extensively orientationally disordered on the lattices and these materials are intrinsically very deformable. As a result, a tiny amount of pressure is able to suppress the extensive orientational disorder to induce the phase transitions to the ordered state and thus huge pressure-induced entropy changes are obtained. These two merits make plastic crystals the best barocaloric materials so far.
This research is the first report that entropy changes can exceed one hundred joule per kilogram per kelvin. It represents the best results among all caloric-effect materials (barocaloric effect as well as its analogies such as magnetocaloric effect, electrocaloric effect, and elastocaloric effect), regarded as a milestone. The microscopic physical scenario established using the neutron scattering technique is helpful for designing even better materials in the future. As far as refrigeration application is concerned, the plastic crystals reported here are very promising given that they are abundantly-available, environmentally-friendly, easy-driven, and high-performance. This work points to a new direction for emerging solid-state refrigeration technologies.
These findings were published in Nature recently. For more details, please refer to the publication at doi: 10.1038/s41586-019-1042-5.
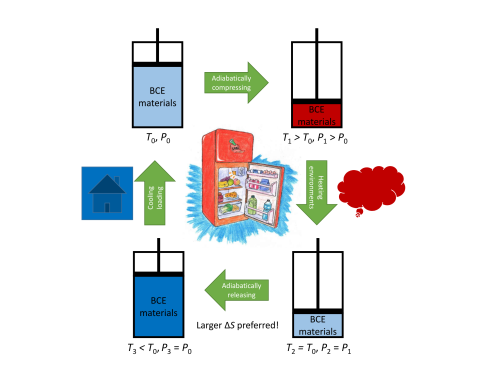
Figure 1. Schematic diagram of the refrigeration cycle based on barocaloric effects. (Image by IMR)
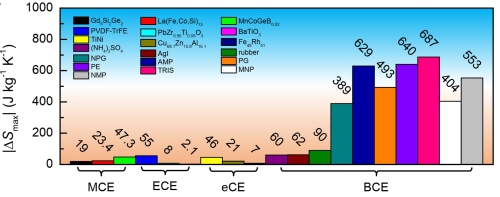
Figure 2. Comparison of the maximum entropy changes of leading caloric materials. MCE: magnetoclaoric effect; ECE: electrocaloric effect; eCE: elastocaloric effect; BCE: barocaloric effect. The plastic crystals identified in the present work are neopentylglycol (NPG), pentaglycerin (PG), pentaerythritol (PE), 2-Amino-2-methyl-1,3-propanediol (AMP), tris (hydroxymethyl) aminomethane (TRIS), 2-Methyl-2-nitro-1-propanol (MNP), 2-Nitro-2-methyl-1,3-propanediol (NMP). (Image by IMR)
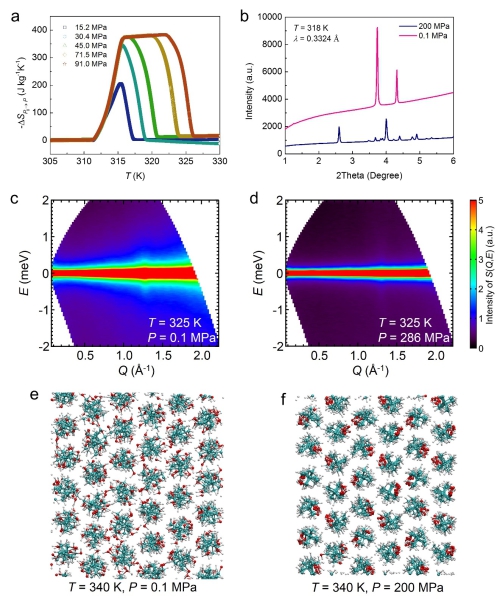
Figure 3. Pressure dependent physical properties of the representative plastic crystal NPG. (a). Pressure-induced entropy changes as a function of temperature under varying pressures. (b). X-ray diffraction patterns, showing a pressure-induced phase transition. (c,d). Neutron scattering spectra, displaying that the pressure suppresses the quasi-elastic signal originating from the molecular orientational disorder. (e,f) Structural snapshots of molecular dynamics simulations, where pressure aligns the molecules. (Image by IMR)
Abbreviations:
1). J-PARC: Japan Proton Accelerator Research Complex) is a high intensity proton accelerator facility. It is a joint project between KEK and JAEA and is located at JAEA Tokai. J-PARC aims for the frontier in materials and life sciences, and nuclear and particle physics. J-PARC uses high intensity proton beams to create high intensity secondary beams of neutrons, hadrons, and neutrinos.
2). SPring-8: SPring-8 (an acronym of Super Photon Ring – 8 GeV) is a synchrotron radiation facility located in Hyōgo Prefecture, Japan, which was developed jointly by RIKEN and the Japan Atomic Energy Research Institute. It is owned and managed by RIKEN, and run under commission by the Japan Synchrotron Radiation Research Institute.
3). ANSTO: The Australian Nuclear Science and Technology Organisation is a statutory body of the Australian government, formed in 1987 to replace the Australian Atomic Energy Commission. Its head office and main facilities are in southern outskirts of Sydney at Lucas Heights, in the Sutherland Shire.
Media links:
New Scientist Phys.org Chemical & Engineering News ORF.at ABC.net.au
Media Contact:
Huang Chengyu
General Office, Institute of Metal Research, Chinese Academy of Sciences
E-mail: cyhuang@imr.ac.cn
Tel: 86-24-23971608