Scientists Visualize the Formation of Intermetallic PdZn in Atomic-scale: Interfacial Fertilization by PdHx
Intermetallic materials are novel and highly efficient heterogeneous catalysts due to their well-defined structures and tunable compositions. However, despite enormous efforts to acquire supported PdZn intermetallic catalysts in a controllable fashion, there is a lack of convincing evidence about the key elementary steps associated with the intermetallic process. Thus, mechanistic clarification and rational design of catalyst are hindered.
Usually, the formation of PdZn was investigated under ultrahigh-vacuum conditions based on some model systems. While considering the very different chemical environment and material gap between the model and real systems, a study on technologically applied PdZn supported catalyst under working conditions is necessary to gain insightful understanding regarding the real intermetallic process. Especially understanding the details about microstructural evolution of the PdZn intermetallic at nano or atomic scales remains challenging.
Furthermore, the role of H2 in formation of the bimetallic alloy has not been rationalized. Visualization of the formation of the active species with high spatial resolution will contribute to fundamental studies as well as practical applications.
Recently, Prof. ZHANG Bingsen ,Prof. SU Dangshengfrom Institute of Metal Research, Chinese Academy of Sciences and their collaborators, studied the PdZn phase transitions under realistic catalytic conditions by using the in-situ TEM gas-heating holder (DENSsolutions Climate) with home-made gas control systems.
They present the first atomic-scale visualization of a complete phase transition from Pd/PdHx to PdZn on a ZnO support under H2 atmosphere, which has been systematically analyzed by integrating in-situ TEM, EELS, and X-ray diffraction (XRD) data.
Significantly, the interfacial PdHx species were generated quickly under an H2 atmosphere and at elevated temperature these species were identified as key intermediates in the following transformation. Subsequently, a consecutive phase transition started at the PdHx/ZnO interface at higher temperature, then proceeded along the PdHx <111> direction until the whole NP was converted to the PdZn structure.
This work not only discloses detailed microstructural information in reference to the catalyst activation, but also sheds light on the rational design and optimum synthesis of intermetallic composite catalysts.
The results were recently published on Angew. Chem. Int. Ed.
This work is supported by the National Natural Science Foundation of China and Youth Innovation Promotion Association CAS.
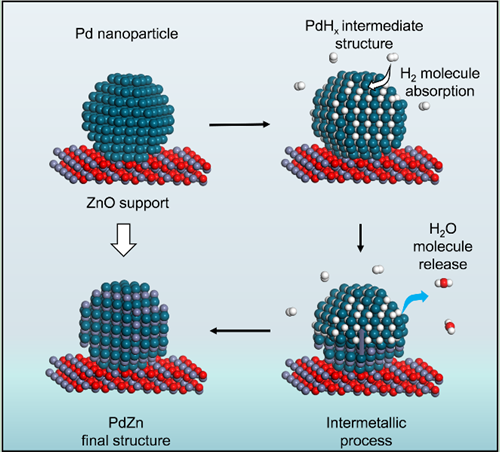
Figure. A diagram depicting the proposed phase-transition process from Pd to PdZn. Atom key: Pd (dark green), O (red), H (white), and Zn (gray). (Image by IMR)
Contact
HUANG Chengyu
E-mail:cyhuang@imr.ac.cn