Researchers Aimed to Develop Chitosan Derivatives with Triple Quaternary Ammonium Groups as Potential Agricultural Fungicides
Recently a research team led by Prof. Li Pengcheng in the Institute of Oceanology of the Chinese Academy of Sciences (IOCAS) have succeeded in synthesizing a novel type of water soluble chitosan derivatives with triple quaternary ammonium groups (TQCSPX). The derivatives have significantly enhanced antifungal activity after quaternized compared with the original chitosan (CS). This findings have been published in International Journal of Biological Macromolecules entitled “Synthesis, characterization and antifungal efficacy of chitosan derivatives with triple quaternary ammonium groups”. The first author of the study was Dr. Liu Weixiang and the corresponding author was Prof. Li Pengcheng.
Plant diseases cause considerable losses to crop yields and limit crop production every year, among them, plant pathogenic fungi are the main pathogens. For example, pepper, tomato and eggplant can be infected by plant pathogens such as Phytophthora capsici; rice and soya can be affected by Rhizoctonia solani; cucumber can be infected by Fusarium oxysporum; many kinds of vegetables and fruits can be infected by Fusarium solani. Fungi cause enormous economic losses to agriculture, and some can even cause human and animal disease. It is necessary to develop a bio-sourced antifungal agent for agricultural use.
Many researches have shown that the antifungal activity of quaternized chitosan derivatives is mainly affected by the positive charge. In that case, the density of positive charge could influence the efficiency of antifungal activities.
In this work, the researchers designed and synthesized two kinds of chitosan derivatives with triple quaternary ammonium groups. They used quantum chemistry calculation method based on the density functional theory (DFT) to explore the possibility of dehydrogenation of the β-C and β-N in IM-1, the results showed that the energy barrier of dehydrogenation reaction on β-C (TS-2’) is lower than that of the dehydrogenation reaction on β-N (TS-2).
It was found that the antifungal activity of TQCSPX significantly increased compared with that of chitosan (CS). Among them, the TQCSPX exhibited relatively high inhibitory effect against P. capsici and R. solani, and the highest antifungal index was 91.94% and 87.18%, respectively. Moreover, at the same doses, the chitosan derivatives exhibited inhibition rates superior to that of Oligosaccharins (a widely used commercially available fungicides) against R. solani. The powerful disruptive effect of aminopyridine-functionalized chitosan on the microorganism was probably based on electrostatic interaction of positively charged moieties of the cationic molecules and negatively charged components on microbial cell membrane. This electrostatic interaction could facilitate changes of permeation property of the membrane wall to provoke internal osmotic imbalances and also cause hydrolysis of the peptidoglycans in the microbial cell wall to the leakage of intracellular electrolytes and nutrients.
The study was supported by the National Natural Science Foundation of China, the Scientific and Technological Innovation Project Financially Supported by Qingdao National Laboratory for Marine Science and Technology and the science and technology program Supported by Qingdao.
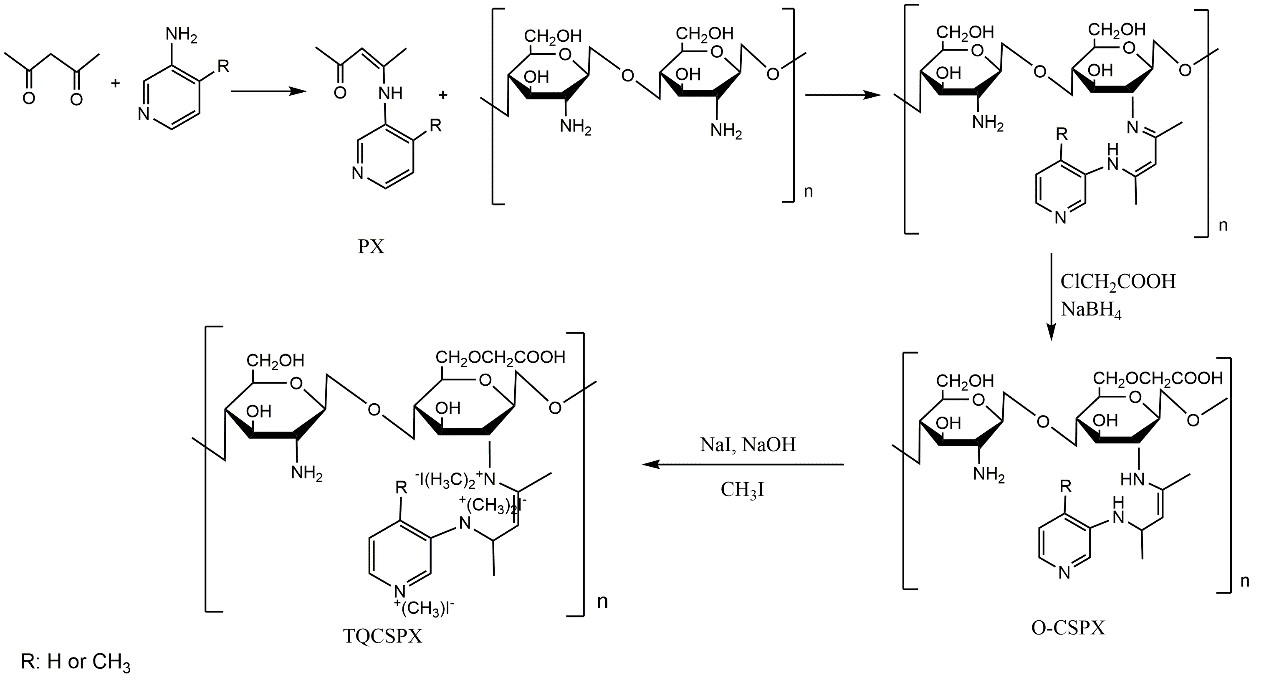
Figure 1. Synthetic route of chitosan derivatives with triple quaternary ammonium groups (TQCSPX)
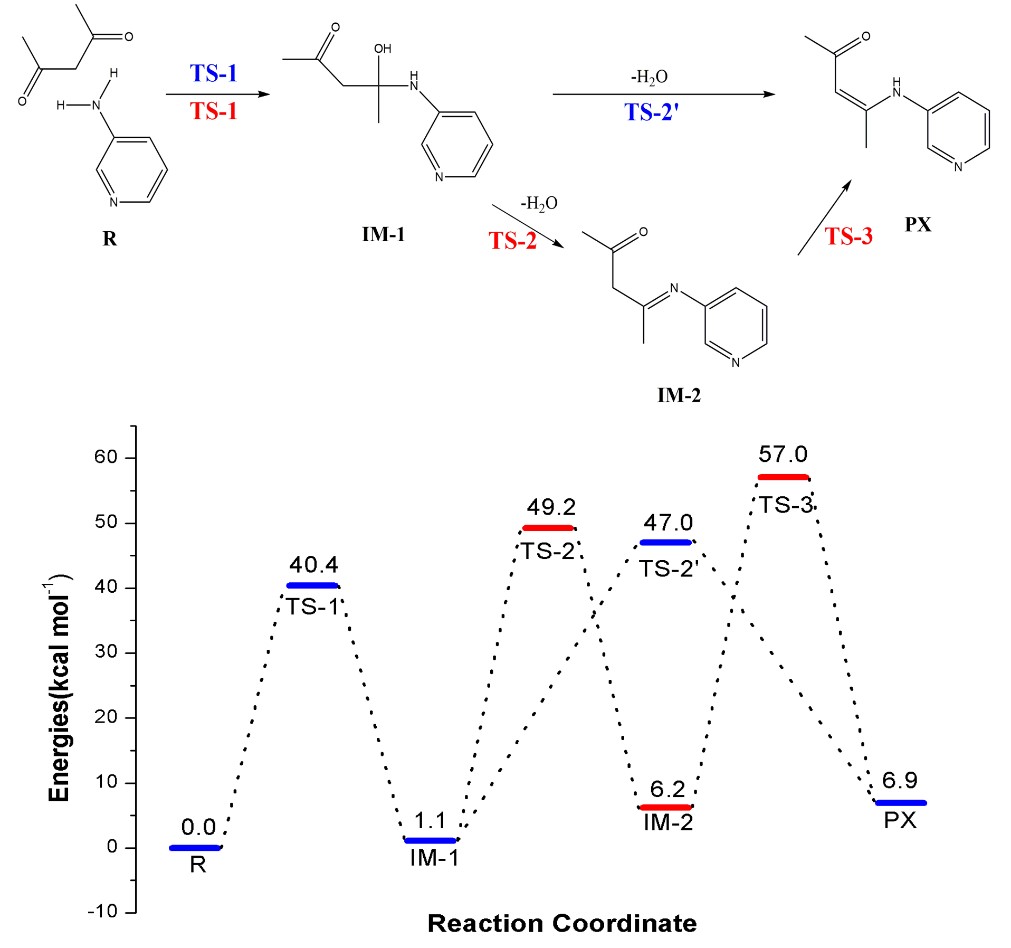
Figure 2. Energy profile of two speculated PX formation mechanisms
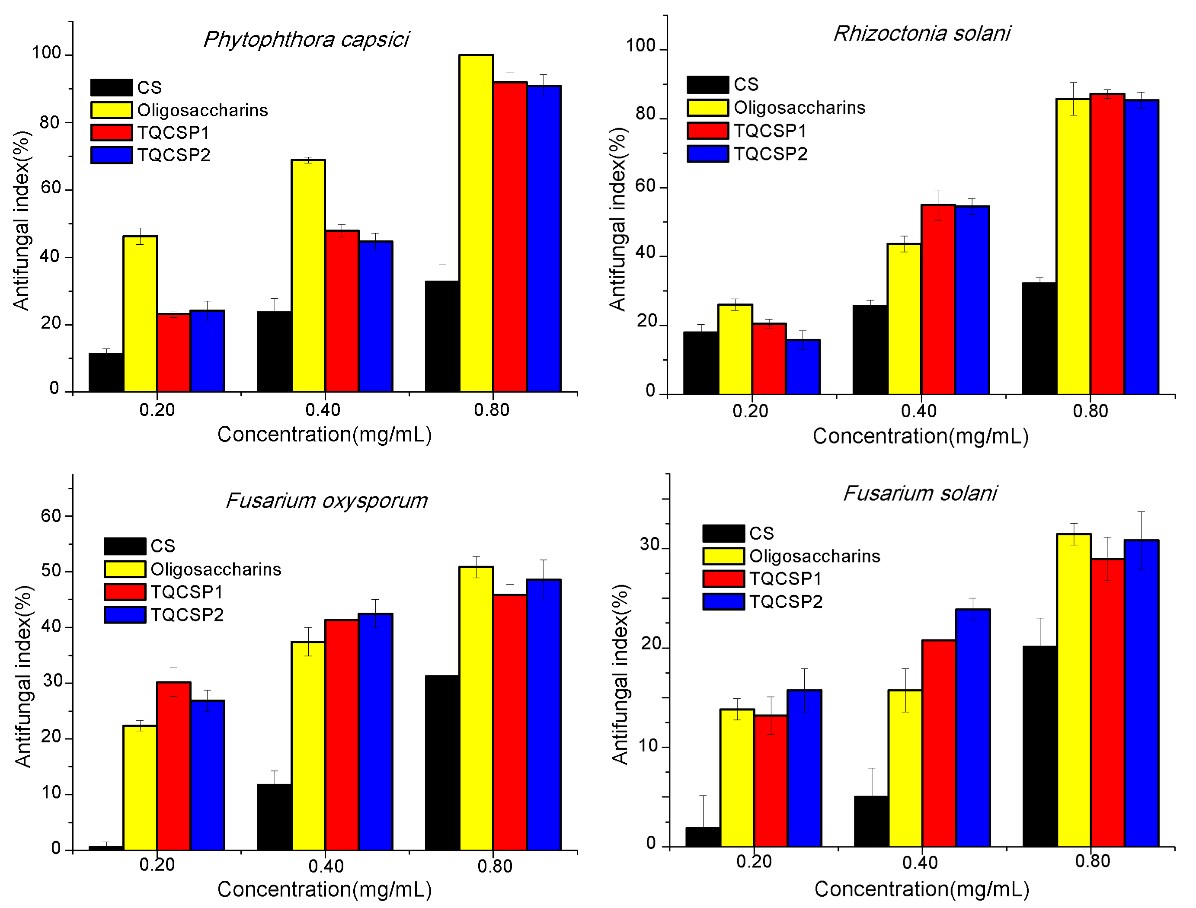
Figure 3. Antifungal activity of chitosan (CS), Oligosaccharins and chitosan derivatives with triple quaternary ammonium groups (TQCSPX) against four selected fungi