Research progress in carbon nanotube confined catalysis
Mr. Fan Zhang, Prof. Xiulian Pan and Prof. Xinhe Bao et al. from the State Key Laboratory of Catalysis have recently made progress in carbon nanotube confined catalysis. The results are published at “Proceedings of the National Academy of Sciences of the United States of America” (PNAS) (http://www.pnas.org/content/ early/2013/08/22/1306784110.full.pdf+html).
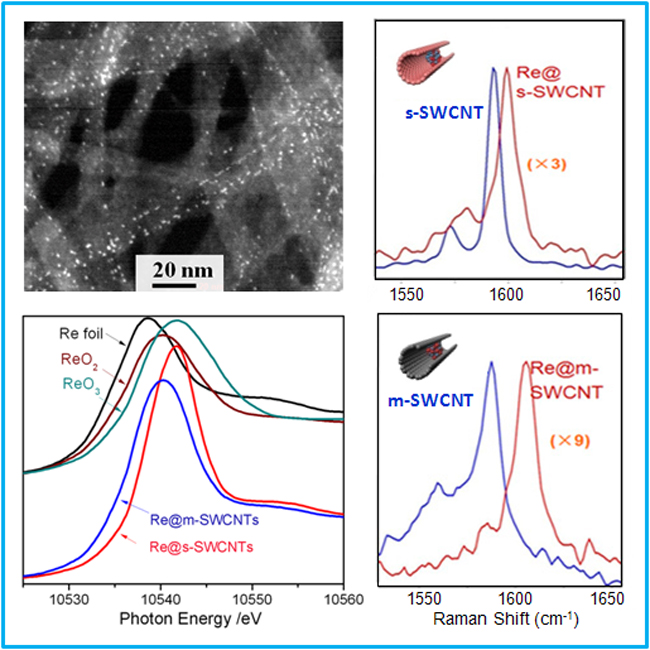
The unique property of carbon nanotube channels has triggered wide research interest in different fields. An increasing number of studies show that confinement of metal or metal oxide nanoparticles inside these channels often leads to significantly modified catalytic activity with respect to the same bare metal nanoparticles or those dispersed on the outer walls. It is demonstrated in this work that reactions can be further modulated by the electronic nature (metallic vs. semiconducting character) of nanotubes by taking encapsulated rhenium nanocatalysts as a probe. Particularly, the chemical state of the encapsulated rhenium is tuned due to host–guest electronic interaction. This is of significance for catalytic reactions sensitive to the chemical state of active metals, because it may change the reaction pathways. This can be exploited for rational design of active catalysts with stable species as a desired valence state can be obtained by selecting specific-type SWCNTs and a controlled thermal treatment.
This work is supported by the Natural Science Foundation of China and the Ministry of Science and Technology. (By Xiulian Pan)