Scientists in DICP developed a new labeling appraoch for quantitative proteomics
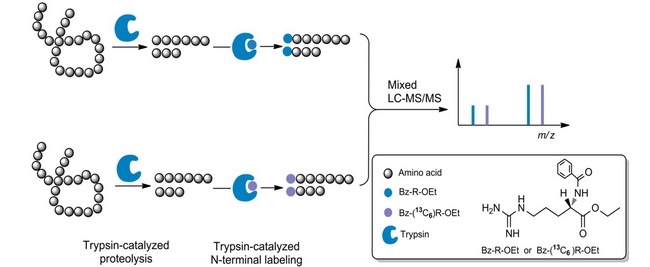
Recently, the research team headed by Prof. Hanfa Zou in CAS Key Lab of Separation Sciences for Analytical Chemistry, National Chromatographic R&A Center, Dalian Institute of Chemical Physics, Chinese Academy of Sciences developed a new N-terminal specific stable isotopic labelling appraoch for quantitative proteomics. This labeling appraoch was achieved by using trypsin as the ligase. This work was published in a new issue of Angew. Chem. Int. Ed. (2013, 52, 9205-9209).
It’s a big chllenge to incorporat isotopic tags to the N termini of peptides without modifying their side chains by chemical methods. To overcome this dificulty, Zou et al. have proposed an enzymatic appraoch. Trypsin, a serine protease, was used as the ligase to catalyze the coupling of arginine derivatives to tryptic peptides in organic solvent. By the formation of a new peptide bond, this new enzymatic labeling approach enabled the specific incorporation of one tag to peptide N-terminal. Additional fragment ions due to the dissociation of the new peptide bond in tandem mass spectrometry greatly improved the peptide identification confidence. Reliable and accurate quantification of proteins were demonstrated by using model proteins as well as proteomics samples. As a specific terminal labeling approach, its high performance in straightforward distinction of N-terminal fragment ions from other ions for de novo peptide sequence was also demonstrated. This study opens up an unique avenue for N-terminal labeling of tryptic peptides without modification of side chains under mild conditions. (By Yanbo Pan)