|
|
A Series of Progress in Copper-Catalyzed Asymmetric Reaction |
| |
Group 02T2 |
ArticleSource: |
大连化学物理研究所 |
Update time: |
2013-12-20 |
|
Recently, a research team headed by Prof. Xiang-Ping Hu in Dalian Institute of Chemical Physics, Chinese Academy of Sciences has reported the first copper-catalyzed enantioselective decarboxylative propargylic alkylation of propargyl-ketoesters with a newly developed tridentate chiral P,N,N-ligand. In this method, the loss of CO2 replaces the need to selectively prepare preformed enolate equivalents, and both the nucleophile and the electrophile are formed in situ in catalytic concentration. The reaction can be performed under the mild condition with high enantioselectivities. This new reaction should provide a complementary strategy for catalytic asymmetric propargylic substitution. The result has been published online in Angew. Chem. Int. Ed. (DOI: 10.1002/anie.201309182). 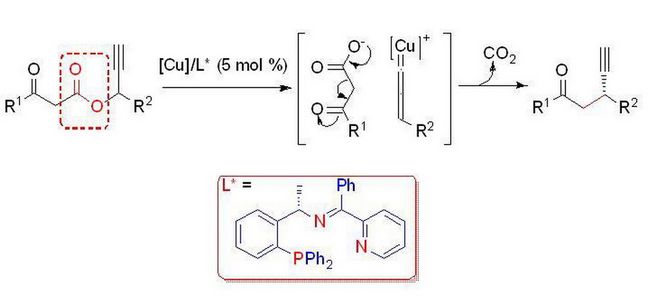
On the other hand, a collaborative team led by Prof. Xiang-Ping Hu from the Dalian Institute of Chemical Physics and Prof. Hong-Chao Guo from the China Agricultural University, has reported the first copper-catalyzed asymmetric [3+3] cycloaddition of azomethineylides with azomethine imines, which led to a variety of optically active hexahydro-8H-pyrazolo[1,2-][1,2,4]triazin-8-one derivatives with potential biological activity. The result has been published in Angew. Chem. Int. Ed. (2013, 52, 12641-12645). These important findings should further broadened the scope of copper-catalyzed asymmetric reactions, and provide some simple and efficient approaches for the construction of complex chiral frameworks.(by Zhu Fulin) |
|
|