|
|
New Progress in the Study of Methanol to Olefins (MTO) Reaction Mechanism |
| |
DNL12 |
ArticleSource: |
大连化学物理研究所 |
Update time: |
2013-11-23 |
|
MTO reaction is a heterogeneous catalytic reaction with a series of complex steps occurrence on acidic zeolites for hydrocarbon formation, for which “Hydrocarbon pool” (HCP) mechanism has attracted extensive attentions. Direct observation, understanding of HCP formation and significance in methanol conversion inside zeolites or silicoaluminophosphate (SAPO) catalysts under real working conditions is still a great challenge. A new progress in methanol to olefins (MTO) reaction mechanism has been achieved recently by Prof. LIU Zhongmin(National Engineering Laboratory for Methanol to Olefins, DICP) in collaboration with Prof. DENG Feng nd Prof. ZHENG Anmin (Wuhan Institute of Physics and Mathematics, CAS). National Engineering Laboratory for Methanol to Olefins focuses on the application and fundamental researches of methanol conversion to light olefins. After the successful observation of heptaMB+ in real MTO reaction systems via employing a newly-synthesized SAPO type molecular sieves with big supercages (J. Am. Chem. Soc.2012, 134 (2), 836–839), a new progress has also been made in the continuous study on the formation and the roles of carbeniums in CHA zeolite, a type of zeolite with great potential in the industrial application. For the first time, two important carbenium ions involved in MTO reaction, heptamethylbenzenium cation (heptaMB+) and pentamethylcyclopentenyl cation (pentaMCP+) have also been directly observed in CHA-type catalysts under the real reaction conditions, in which the pentaMCP+ is a newly-observed carbenium ion in the MTO reaction. The assignments of the two carbenium ions were confirmed by the combined techniques of NMR, GC-MS and theoretical calculation. 13C MAS NMR measurements and isotopic switch experiments provide substantial proofs that both pentaMCP+ and heptaMB+ are important intermediates for the MTO reaction. The theoretical prediction of two catalytic cycles following paring and side-chain methylation mechanisms demonstrated that both of them are energetically feasible reaction routes while the side-chain methylation mechanism is more predominant due to the lower energy barrier. The relationship between stability and reactivity of the two carbenium ions has also been discussed in detail. These results were published in Angew. Chem. Int. Ed. (Angew. Chem. Int. Ed.2013, 52(44), 11564-11568) This work has been supported by the National Natural Science Foundation of China andStrategic Priority Research Program of CAS. (by XU Shutao and WEI Yingxu) 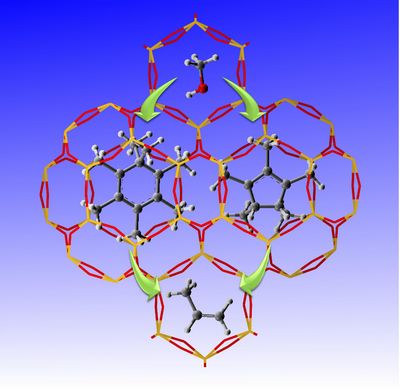 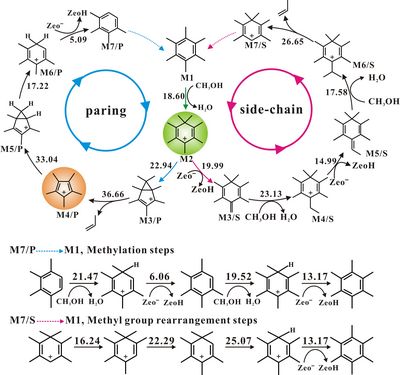
Fig. Two carbenium ions confined in CHA nanocage during MTO reaction process (left) & Catalytic cycles of the paring and side-chain reaction mechanisms for MTO reaction (right). Contact: Prof. Zhongmin Liu Email: liuzm@dicp.ac.cn |
|
|