Based on the comprehensive first-principle kinetics study, Mr. LIU Jinxun, Dr. SU Haiyan and Prof. LI Weixue of Dalian Institute of Chemical Physics (DICP), has made progress in the investigation of the structure sensitivity for syngas (CO and H2 mixture) conversion recently. They found that cobalt crystallographic structure plays a decisive role on C=O dissociation for the first time. The insights revealed would be valuable for synthesizing better, stable catalysts with maximum mass-specific reactivity. The work was published as a communication on Journal of the American Chemical Society ( J. Am. Chem. Soc. 135 (2013) 16284–16287 ) .
The structure sensitivity of catalytic reactions and the stability of the catalysts with maximum mass-specific reactivity are the one of the key issues in heterogeneous catalysis. One classic example is cobalt-catalyzing Fischer–Tropsch synthesis (FTS) converting CO and H2 from coal, natural gas and biomass to valuable liquid fuels and chemical products. For a long time, it has been observed experimentally that the corresponding activity is closely related to the Co crystallographic structure. Specifically, the catalyst with more hexagonal close-packed (HCP) Co would have a higher FTS activity than that with more face centered-cubic (FCC) Co. Nevertheless, it remains open as to whether and why HCP Co catalysts have higher FTS activity than FCC ones. Meanwhile, HCP Co would transform to FCC Co with decreasing particle size and increasing temperature under realistic operating conditions. These prevent greatly rational of the design and optimization of highly efficient yet stable Co catalysts.
Using C=O activation in the presence of hydrogen as a probe, the group led by Prof. LI conducted a comprehensive first-principle kinetic study and found for the first time that cobalt crystal phase plays a decisive role in C=O dissociation: (1) HCP Co has higher intrinsic activity than FCC Co, and the catalytic activity is strongly dependent on the morphology of HCP Co. (2) CO activation prefers the direct dissociation pathway on HCP Co while H-assisted CO dissociation on the FCC Co. The origin is identified from the lower symmetry of HCP Co, a fact of that allows exposing various denser yet favorable active sites and facets not available for FCC Co.
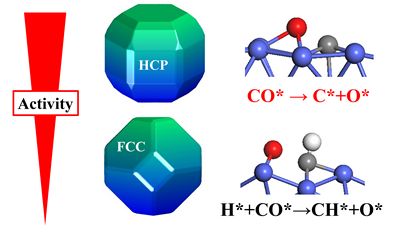

Based on the theoretical findings, the specific structure with higher mass specific activity by exposing highly active HCP Co(10-11) facet, without necessarily decrease of the catalyst size, was suggested. The great influence of crystallographic structure and corresponding morphology effect on formation of the active sites with higher intrinsic activity and density was illustrated in this study. The insights revealed might open up a new avenue for the design of better, stable catalysts with maximum mass-specific reactivity, in which ab initio calculation and material synthesis would play an essential role.
This work was supported by Distinguished Young Scholar Funding from Natural Science Foundation of China and Ministry of Science and Technology of China. (by LIU Jinxun and SU Haiyan)