C-H activation has been employed as a powerful strategy in synthetic organic chemistry. In recent years, with their unique structural characteristics and organometallic properties, rhodium(III)-Catalyzed C-H activation has been increasingly explored, leading to various novel synthetic methods. Thus rhodium(III) catalysis stand out with high activity, broad substrate scope, high functional group compatibility, and mild reaction conditions. Directed by Prof. Xingwei Li, the 02T3 group has carried out systematic and extensive studies in this field since 2010. In this year, three research articles have been published/accepted in Angew. Chem. Int. Ed. (Angew. Chem. Int. Ed. 2013, 52, 2577; Angew. Chem. Int. Ed. 2013, 52, 8995; Angew. Chem. Int. Ed. 2013, DOI: 10.1002/anie.201305902.)

Reports on the coupling of strained ring substrates with arene substrates are limited. The 02T3 group and the 202 group collaboratively developed [RhCp*Cl2]2/AgSbF6-catalyzed and chelation-assisted insertion of aryl C-H bond into aziridines, leading to the synthesis of b-branched ethylamines. The multifold role of AgSbF6 has been explored and a key intermediate of 8-membered rhodacyclic complex has been isolated as a result of the insertion of the Rh-C bond into the aziridine ring. A plausible mechanism has been proposed on the basis of a series of mechanistic studies.

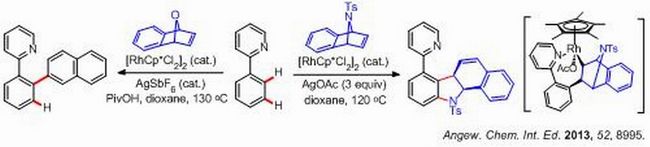
Inspired by the result of this strained ring substrate, the 02T3 group further extended the ring to 7-oxa/azabenzonorbornadienes, which are strain-activated internal alkenes that have found wide applications in synthesis. The coupling of 2-phenylpyridines with 7- azabenzonorbornadienes afforded dihydrocarbazoles under oxidative conditions as a result of twofold C-H activation. Mechanistic studies indicated that the first C-H activation (ortho C-H bond) is rate-limiting and a key Rh(III) intermediate has been isolated as a result of the migratory insertion of the Rh-C bond into the olefin. In contrast to the oxidative coupling of 7-azabenzonorbornadienes, 7-oxabenzonorbornadienes only underwent redox-neutral dehydrative coupling with 2-phenylpyridine to give the 2-naphthylation product.
Previously, the coupling partners to arene C-H bonds are mostly limited to unsaturated bonds or strained rings when catalyzed by Rh(III) complexes. The 02T3 group recently achieved an important C-H azidation reaction of arenes, where NaN3 was used as a common azidating reagent and a hypervalent iodine as an efficient oxidant. This reaction is highly attractive in that organic azides have found wide applications in synthetic chemistry, material, and biological studies. The adization product can be further readily functionalized. Mechanistic studies revealed that no radical species is likely involved, which is in sharp contrast to those related systems previously reported. In addition to azidation, nitration of the same substrate has also been realized using NaNO2 under modified conditions.

These important findings have broadened the scope and applicability of metal-catalyzed C-H activation in synthetic organic chemistry, and these results should cast light on rational design and understanding of new catalytic C-H activation systems (By Xingwei Li).